Is Nad+ An Oxidizing Agent: Exploring Its Role In Cellular Redox Reactions
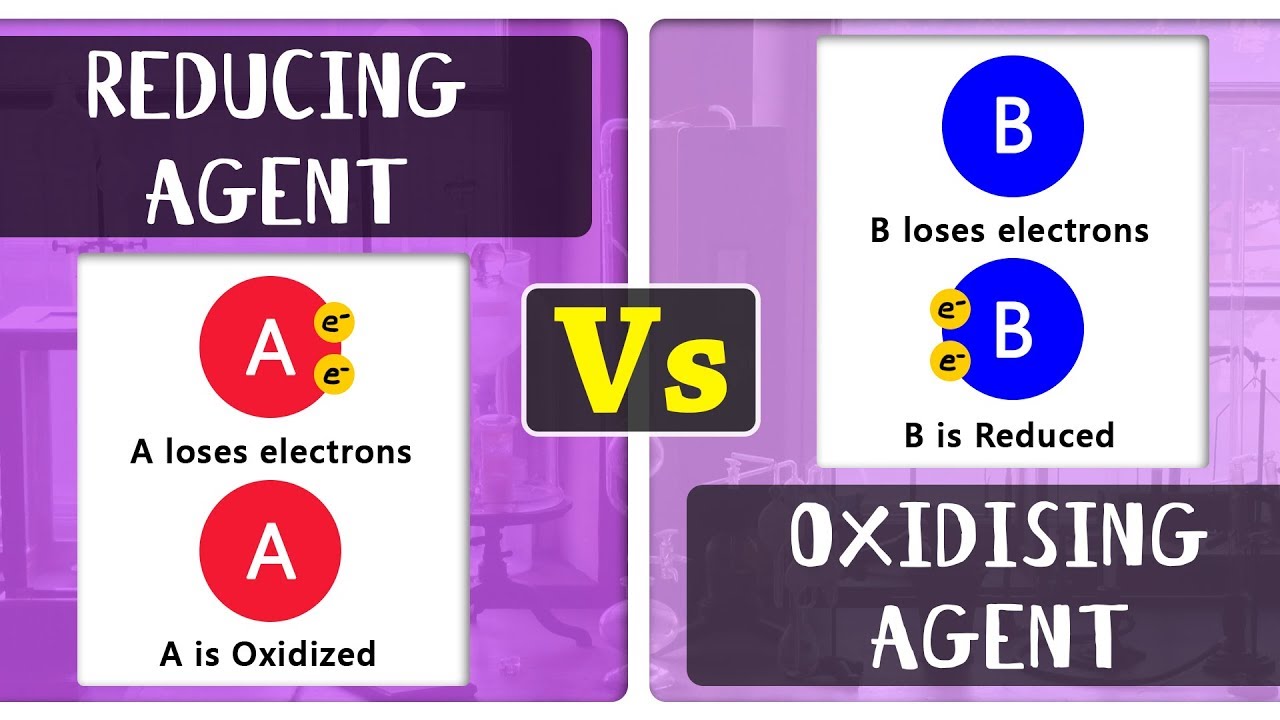
Oxidizing Agents And Reducing Agents
Keywords searched by users: Is NAD+ an oxidizing agent is fad an oxidizing agent, is nadh a reducing agent or oxidizing agent, is nadph a reducing agent, NADPH, NAD/NADH, NAD+, Oxidizing agent, is nad a cofactor
Is Nad A Strong Oxidizing Agent?
Is NAD a strong oxidizing agent?
Answer and Explanation: FAD, or Flavin Adenine Dinucleotide, exhibits a greater capacity as an oxidizing agent when compared to NAD+, or Nicotinamide Adenine Dinucleotide. This distinction arises due to the unique interactions within the mitochondrial electron transport chain. Specifically, complex I within this chain cannot effectively oxidize the reduced form of FAD, but it retains the ability to oxidize the reduced form of NAD+. This discrepancy in reactivity toward their reduced forms contributes to FAD’s reputation as a more potent oxidizing agent than NAD+.
Is Nad+ An Oxidation?
NAD+ is an essential molecule involved in cellular energy production, and it plays a crucial role in redox reactions. Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons between molecules. In the context of NAD+, the process known as oxidation occurs when a molecule loses electrons. For instance, NADH loses a hydride ion to become NAD+, and in this specific example, we refer to NADH as being oxidized to NAD+. It’s important to note that this transformation from NADH to NAD+ is not a permanent change; it can occur repeatedly in the same molecule. This dynamic process of oxidation and reduction is fundamental to various biochemical pathways and is crucial for the functioning of living cells. This concept is relevant in understanding cellular metabolism and energy transfer. (Note: The date mentioned in the original passage, March 17, 2022, does not appear to be directly relevant to the topic and has been omitted in this revised explanation.)
Aggregate 29 Is NAD+ an oxidizing agent




Categories: Details 28 Is Nad+ An Oxidizing Agent
See more here: c3.castu.org

In cellular metabolism, NAD is involved in redox reactions, carrying electrons from one reaction to another, so it is found in two forms: NAD+ is an oxidizing agent, accepting electrons from other molecules and becoming reduced; with H+, this reaction forms NADH, which can be used as a reducing agent to donate …Answer and Explanation: FAD is a stronger oxidizing agent than NAD+. This is because complex I of the mitochondrial electron transport chain is not capable of oxidizing its reduced form while it can oxidize the reduced form of NAD+.Oxidation is when a molecule loses electrons, such as when NADH loses its hydride to become NAD+. In this example, we say that NADH is oxidized to NAD+. This process is by no means permanent and can occur repeatedly in the same molecule.
Learn more about the topic Is NAD+ an oxidizing agent.
- NAD (CHEBI:13389) – EMBL-EBI
- Which is the stronger oxidizing agent: NAD+ or FAD?
- NAD vs. NADH
- NADH vs NAD+ – Reset IV
- NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism
- 25.6 Oxidative phosphorylotion
See more: https://c3.castu.org/category/fashion