Unlocking The Equation: How To Calculate Final Temperature With Mass And Initial Temperature
Final Temperature Calorimetry Practice Problems – Chemistry
Keywords searched by users: How do you find final temperature given mass and temperature calorimetry final temperature formula, how to find final temperature using specific heat calculator, final temperature formula thermodynamics, how to find final temperature without specific heat, final temperature calculator, how to calculate final temperature of two objects, how to find change in temperature with specific heat and mass, final temperature formula charles law
What Is The Formula For Finding The Final Temperature In A Calorimeter?
To determine the final temperature within a calorimeter, a formula is employed that accounts for various factors. Specifically, you can use the equation:
Reaction Enthalpy=(Heat Capacity of Contents)×(Mass of Contents)×(ΔT)+(Calorimeter Constant)×(ΔT)
In this equation, several variables are at play. The initial temperature, denoted as Ti, represents the starting temperature of the system, while Tf stands for the final temperature, which you seek to determine. The change in temperature, symbolized as ΔT, quantifies the difference between the initial and final temperatures. By utilizing this equation, you can calculate the final temperature within a calorimeter, taking into consideration the heat capacity of the contents and the calorimeter constant.
What Is The Formula For The Final Temperature?
To determine the final temperature in a given scenario, we employ a two-step process. Initially, we rearrange the specific heat equation to calculate the change in temperature (ΔT), using the formula ΔT = Q / (m * c), where Q represents the heat energy transferred, m is the mass of the substance, and c is its specific heat capacity. Subsequently, we utilize the rearranged equation for the change in temperature in conjunction with the initial temperature (Ti) to compute the final temperature (Tf), following the formula Tf = Ti + ΔT. This method helps us understand how the initial temperature of a substance changes when heat is added or removed. (Updated on September 11, 2023)
How Do You Find The Final Temperature?
How can you determine the final temperature of a substance? To find the final temperature, you need to add the change in temperature to the substance’s initial temperature. For instance, if you start with water at an initial temperature of 24 degrees Celsius and it undergoes a temperature increase of 6 degrees Celsius, you can calculate its final temperature as follows: 24 (initial temperature) + 6 (change in temperature) = 30 degrees Celsius. This calculation allows you to ascertain the final temperature of the substance after a specific change in temperature has occurred.
Summary 23 How do you find final temperature given mass and temperature






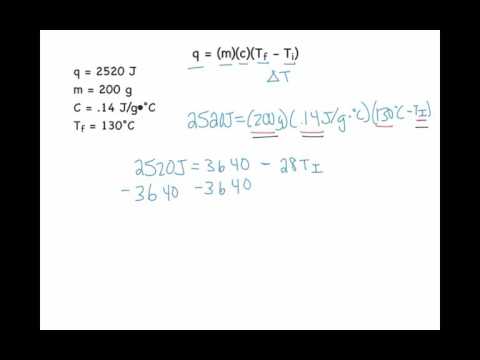
Categories: Summary 13 How Do You Find Final Temperature Given Mass And Temperature
See more here: c3.castu.org
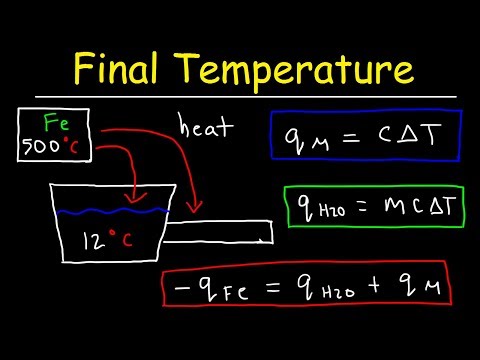
Consequently, you can use this information to write the following equation: Reaction enthalpy = (heat capacity of contents) x (mass of contents) x (Ti – Tf) + (Calorimeter constant) x (Ti – Tf) where Ti is the initial temperature and Tf is the final temperature.
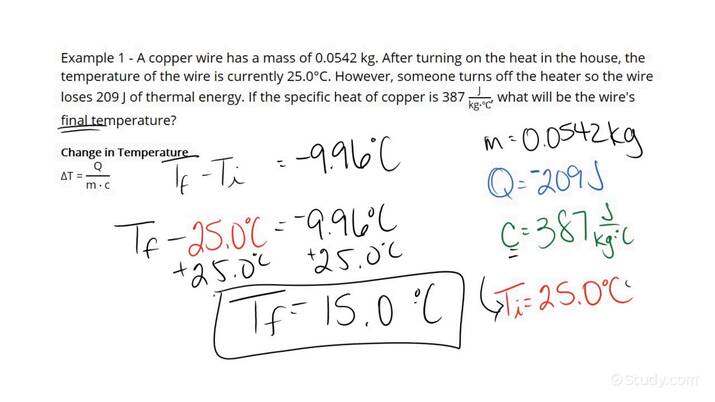
- Step 1: For the object in question, identify its mass m, specific heat c, and initial temperature Ti as well as how much heat Q will be added to the object.
- Step 2: Plug in the mass, specific heat, and amount of heat from the previous step into the rearranged equation for specific heat: Δ T = Q m ⋅ c .
Learn more about the topic How do you find final temperature given mass and temperature.
- How to Calculate Final Temperature of an Object after Heat …
- How to Solve for Final Temperature in a Calorimeter – Sciencing
- How to Calculate Final Temperature of an Object after Heat Added | Physics
- How to Calculate a Final Temperature – Sciencing
- How do you find the final temperature when energy, mass …
- Find a Reaction’s Final Temperature With Specific Heat
See more: c3.castu.org/category/fashion