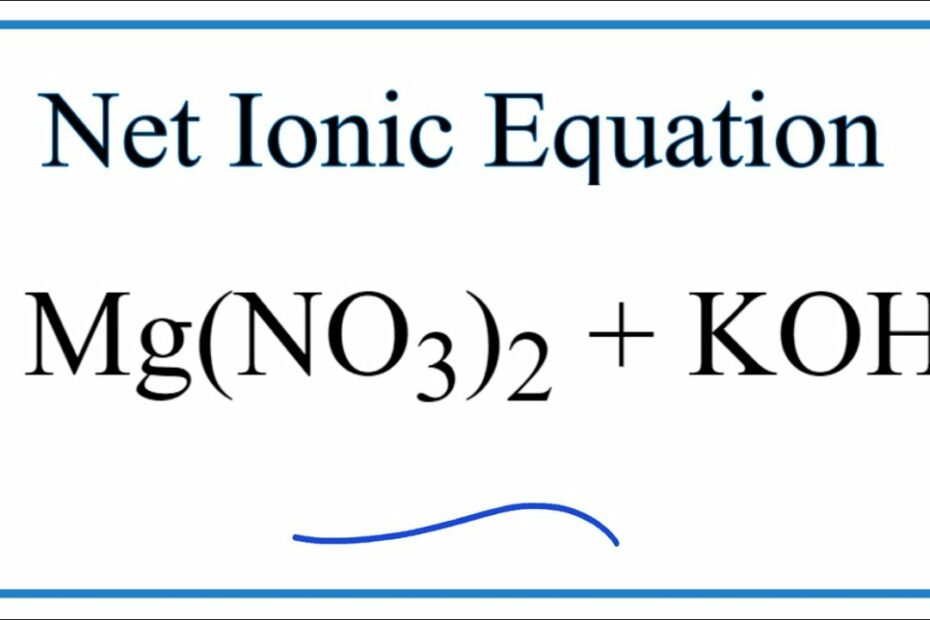What Is The Product Of Koh Mg No3 2: A Chemical Reaction Explained
How To Write The Net Ionic Equation For Mg(No3)2 + Koh = Kno3 + Mg(Oh)2
Keywords searched by users: What is the product of KOH mg NO3 2 koh + mg(no3)2 precipitate, koh+mg(no3)2 net ionic equation, mg(no3)2 + koh pt ion, cuso4 mg(no3)2, k3po4 và ba(no3)2, will koh and mg(no3)2 produce a precipitate, lioh + fe(no3)3, sodium sulfate + copper (ii) nitrate
What Is The Product Of Mg No3 2?
Magnesium nitrate, represented by the chemical formula Mg(NO3)2, is a prominent chemical compound that takes the form of a white crystalline solid. This compound is a result of the combination of magnesium (Mg) and nitrate (NO3) ions. It is important to note that magnesium nitrate, like many nitrates, is highly soluble in water and is often utilized in various industrial and laboratory applications. Understanding the product of Mg(NO3)2 involves recognizing the composition of magnesium and nitrate ions, which combine to form this particular compound, and its physical characteristics as a white crystalline solid.
Does Koh Aq And Mg No3 2 Aq Produce A Precipitate?
Question: Will the combination of Koh (potassium hydroxide, an aqueous solution) and Mg(NO3)2 (magnesium nitrate, also in an aqueous solution) result in the formation of a precipitate?
Answer and Explanation: In this experiment, we are investigating the potential formation of a precipitate by mixing potassium hydroxide (KOH) and magnesium nitrate (Mg(NO3)2), both of which are initially in aqueous form. When these two solutions are combined, a chemical reaction takes place. Specifically, magnesium hydroxide (Mg(OH)2) precipitates out of the solution, while potassium nitrate (KNO3) remains in soluble form. So, to answer the question, yes, the combination of KOH and Mg(NO3)2 does indeed produce a precipitate, which is magnesium hydroxide (Mg(OH)2). This precipitation occurs because magnesium hydroxide is only sparingly soluble in water, leading it to form solid particles, while potassium nitrate remains fully dissolved.
Share 32 What is the product of KOH mg NO3 2
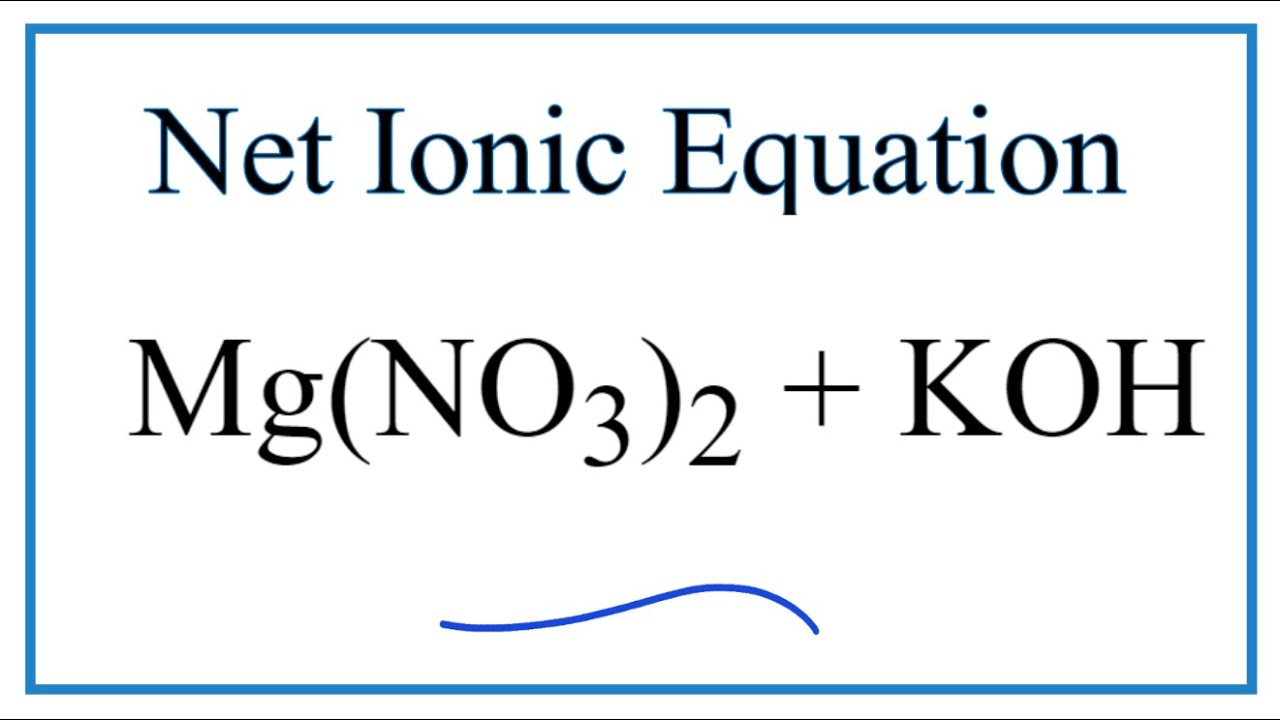






Categories: Collect 48 What Is The Product Of Koh Mg No3 2
See more here: c3.castu.org

Learn more about the topic What is the product of KOH mg NO3 2.
- Mg(NO3)2 + KOH = Mg(OH)2 + KNO3 – Chemical Equation …
- Mg(NO3)2 + KOH → Mg(OH)2 ↓ + KNO3
- Mg(NO3)2 + 2 KOH → Mg(OH)2 + 2 KNO3
- Magnesium nitrate | Mg(NO3)2 | CID 25212 – PubChem
- What is net ionic equation for Mg(NO_{3}) _{2} + KOH?
- Magnesium nitrate (Mg(NO3)2) – Structure, Molecular Mass … – BYJU’S
See more: c3.castu.org/category/fashion