Which Metals React With Hcl: A Comprehensive Guide
Predicting If Hcl Will Dissolve A Metal (Spontaneous Redox Reaction)
Keywords searched by users: Which metals will dissolve in HCl does ag dissolve in hcl, will cd dissolve in hcl, does au dissolve in hcl, does cu dissolve in hcl, what metals dissolve in sulfuric acid, what metals react with hydrochloric acid to produce hydrogen gas, what acid can dissolve metal, can hydrochloric acid kill you
What Metals Will Not Dissolve In Hcl?
Which metals remain unreactive when exposed to dilute hydrochloric acid (HCl)? Gold and copper fall into this category, as they do not undergo chemical reactions with HCl. Conversely, metals like zinc and magnesium readily react with HCl, forming metal salts and releasing hydrogen gas in the process. This distinction highlights the varying reactivity of metals when subjected to hydrochloric acid, with gold and copper remaining inert while zinc and magnesium display a noticeable chemical response.
Can All Metals React With Hcl?
Is it possible for all types of metals to undergo a chemical reaction when exposed to hydrochloric acid (HCl)? When concentrated hydrochloric acid comes into contact with certain metals, it can dissolve them, resulting in the formation of oxidized metal chlorides along with the release of hydrogen gas. However, it’s important to note that not all metals exhibit this reaction with HCl.
Can Hcl Break Down Metal?
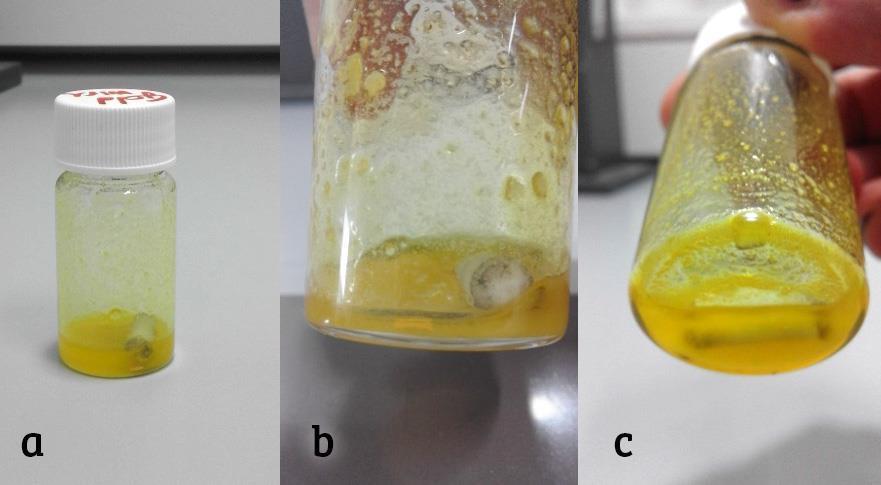
Can hydrochloric acid (HCl) effectively corrode metals? Aqueous solutions of hydrochloric acid have the ability to chemically react with and corrode a wide range of metals. However, it’s important to note that there are exceptions to this reactivity. Metals such as mercury, silver, gold, platinum, tantalum, and certain alloys are resistant to the corrosive effects of hydrochloric acid. In some cases, the acid solution may take on a yellow color due to the presence of trace amounts of iron, chlorine, or organic matter. It’s also important to mention that hydrochloric acid is a non-flammable gas. This information is accurate as of June 30, 2022.
Update 31 Which metals will dissolve in HCl




Categories: Update 87 Which Metals Will Dissolve In Hcl
See more here: c3.castu.org

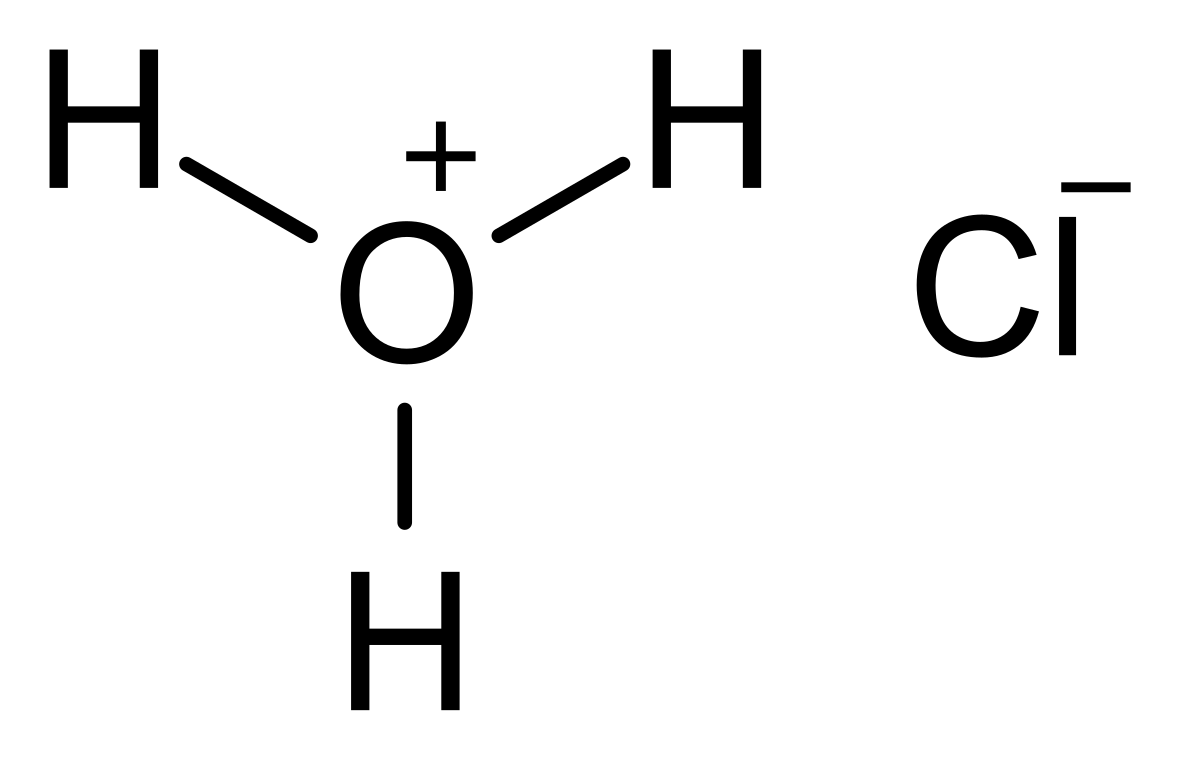
Hydrochloric acid dissolves the less active metals, such as zinc and magnesium, easily. It dissolves the more-resistant iron, copper and related metals less easily, or not at all. Other chemicals, such as nitric acid, will dissolve some metals that hydrochloric acid will not.Therefore, gold and copper will not react with dilute hydrochloric acid while zinc and magnesium will react that will result in the formation of respective metal salts along with the evolution of hydrogen gas.Concentrated hydrochloric acid dissolves many metals and forms oxidized metal chlorides and hydrogen gas but it does not react with all metals.
Learn more about the topic Which metals will dissolve in HCl.
- Advanced STEM Club: Dissolving Metal
- Which of the following metals will not react with dilute hydrochloric acid …
- All metals on reaction with HCl liberate H2 gas. – Toppr
- Hydrochloric acid – DCCEEW
- Does hydrochloric acid dissolve plastic? – Vedantu
- Explain what happens when Aluminium reacts with dilute …
See more: c3.castu.org/category/fashion